I’m finalizing a chapter in my soon-to-be-released book about how the PREP Act was used to throw Americans injured by the COVID-19 jab under the bus. While doing some deep investigation into the Vaccine Adverse Event Reporting System (VAERS) reporting system, co-monitored by the CDC and the FDA, I found that the FDA also has a reporting system to collect adverse reactions to biologics and prescription medication.
In 1969, the FDA established the Spontaneous Reporting System (SRS). This system was designed to allow healthcare professionals, manufacturers, and the public to report adverse drug reactions (ADRs) to medications. The system collected data to help identify safety concerns and detect unanticipated side effects that might not have been observed during clinical trials.
As technology advanced, the SRS was replaced by the FDA Adverse Event Reporting System (FAERS) in 1997. Like the VAERS database created as part of the National Childhood Vaccine Injury Act of 1986, FAERS is a voluntary, self-reporting system. Reports to the FAERS can be made by manufacturers, healthcare professionals, patients, families, and caregivers. If a manufacturer receives a report from a healthcare professional or a consumer, they are required by FDA regulations to report it to the FDA.
The FAERS website admits this about this data:
FAERS data does have limitations.
There is no certainty that the reported adverse event or medication error was due to the product.
FDA does not require that a causal relationship between a product and event be proven.
Reports do not always contain enough detail to evaluate an event properly.
The FDA does not receive reports for every adverse event or medication error that occurs with a product.
Duplicate reports can occur if the same report was submitted by a consumer and by the healthcare provider.
Therefore, FAERS data cannot be used to calculate the incidence of an adverse event or medication error in the U.S. population.
While the total number of reports filed is aggregated and visible to the public, searching the database is not easy. The data can only be accessed by looking at one drug at a time. The database spreadsheets are expansive and challenging to use. After spending about an hour clicking through the available search buttons, I found some information of interest.
Storewide Sale until December 2, 2024
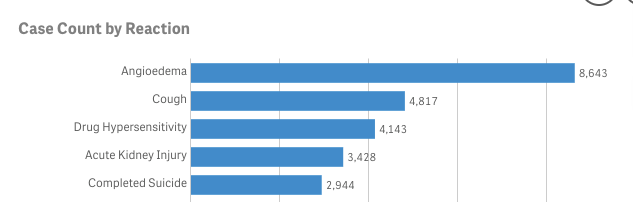
As an example, I searched the database for lisinopril, the most commonly prescribed medication for high blood pressure in America. Classified as an ACE inhibitor, approximately 82.5 million prescriptions for lisinopril have been written as of 2022. This makes it one of the most frequently prescribed medications in the country, ranking second among all drugs, with Synthroid (for low thyroid condition) being #1 and Lipitor is listed as #3.
Since its approval in 1987 as Zestril, 59,370 adverse events have been reported to FAERS, with 14.5% of reactions reported as angioedema, a condition marked by swelling around the lips, eyes, throat, and extremities. In severe cases, it can affect the throat, leading to difficulty breathing, which can be life-threatening.
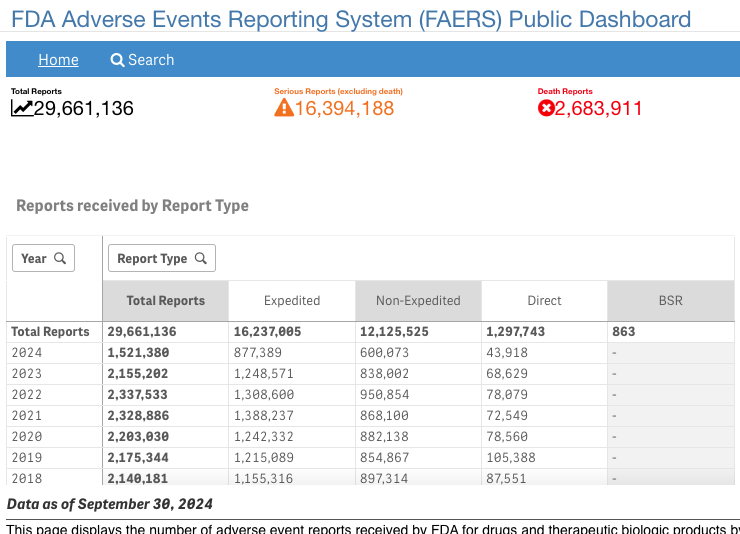
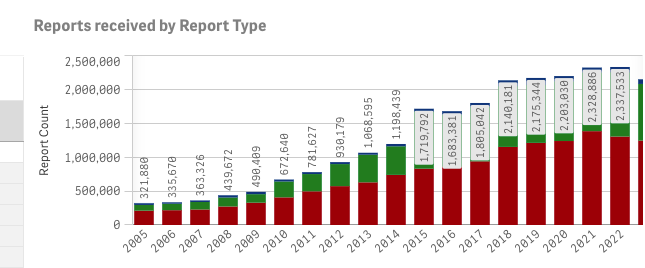
The total number of reports submitted to the FAERS database since 1968 (which includes SRS data) is just over 29.6 million. As recently as 2017 an article published in Expert Opinion of Drug Safety estimated that only about 20% of adverse events are reported to this database.
So, if only 1% of vaccine adverse events are reported (NOTE: 1,700,000 reports have been submitted to VAERS regarding the COVID -19 jabs) and only about 20% of drug adverse events are reported, how many people have suffered or died at the hands of the pharmaceutical companies?
When reviewing the data submitted, the reports are termed "expedited," "non-expedited," "direct," and “BSR.” These designations refer to different categories of reporting timelines and actions for adverse events related to drugs or biologics:
1. Expedited Reports: These are reports involving serious adverse events or new safety concerns that are not yet described in the product's labeling. Due to their urgency, they require fast action from the manufacturer to inform the FDA and the public.
2. Non-expedited Reports: These typically involve less serious adverse events or situations where the event has already been well-documented in the product's labeling. Non-expedited reports are generally submitted quarterly for the first three years following a product's approval and annually afterward. These reports do not require immediate attention but are still part of ongoing monitoring.
3. Direct Reports: These are reports submitted directly to the FDA, bypassing the manufacturer. They may come from healthcare providers, patients, or other sources and are not part of the normal manufacturing report flow.
4. BSR refers to as the "Biologics Safety Report." This term manages reports related to biologics (drugs or therapies derived from living organisms), such as gene therapies and monoclonal antibodies.
Before COVID, few actually knew about VAERS and VAERS reporting. How many know about FAERS and how to report a reaction to a medication? Only a tiny fraction of the number of reports submitted are done so by a patient or their doctor.
When are ALL the drug companies and their physician minions going to be held accountable for poisoning us, from birth to grave, in the name of Health?







I think there will be an accountability. I think millions of people will make sure of it. That’s why it’s important to get copies of all your records cause payday is coming. I know money will not replace your loved ones or your injury…but it will damn sure put a big damper in their lives. Bankrupt their scum asses. Civil lawsuits have come, are coming and will continue to come and you won’t be successful unless you have your medical documentation. Medical records are destroyed after ten years. Make them pay! Get your records now, stay alert in lawsuits, contact counsel to do an assessment of your records by a qualified nurse via report and don’t give up! Do NOT give up! No statute of limitations on murder! Get your records now, they cannot deny your records and if you’re the executor/executrix of a deceased family member, get them asap! Make them pay and pray 🙏🏽
Thank you Dr Tenpenny for highlighting this extremely important info! While I’m certain that RFKjr and others will be appointed and reform healthcare going forward, I hope and pray the killed and injured are acknowledged and those responsible are held accountable.