Originally posted Jan 2023.
The medical community and the media hang their hats on the use of ‘double-blind, placebo-controlled, peer-reviewed studies published in legacy journals such as The New England Journal of Medicine (NEJM) and the Journal of the American Medical Association (JAMA). In a future substack, I will go into detail about the fallacies, and even the scam, of peer review and why it should not be held out as sacrosanct.
For today’s discussion, let’s examine why all vaccine research should be questioned. Yes, ALL of it. If you read enough studies, you’ll see the patterns described below. For this substack, I’ll use this study on the safety of hepatitis B vaccination in children in India as an example. The vaccine used, Revac-B, contained both 0.5mg of aluminum and 0.05 mg of thimerosal, considered to be safe.
1. Vaccine trials can be quite small and include only healthy children.
Every study begins with ‘selection criteria’ that describe including only healthy individuals. This is from the hepatitis B study example:
All 60 subjects included in the study were in good health and had a negative history of hematological, renal, hepatic, or allergic diseases. All were screened and found to have normal blood panels, including normal liver enzymes.
When a vaccine trial has been completed and the vaccine is approved for use by the FDA, the vaccine is recommended for ALL children, regardless of their health condition, family history, or genetics. In fact, the new shot is most ardently pushed on children with underlying health concerns, such as seizure disorders, cardiac anomalies, and conditions such as cystic fibrosis or Down’s syndrome. These children become the next round of experimentation because the vaccines were never tested for safety on these groups and others.
2. Vaccine studies follow side effects for a short period of time.
Most clinical trials monitor for side effects for a paltry 21 days, often less. In some studies, such as in the example we are using, children were monitored for 5 days by study monitors and 5 days by cards given to parents. If no reactions occur, the shot is deemed to be ‘safe.’
However, it can take weeks to months for immune and neurological complications to appear. These arbitrary deadlines, allowed by the FDA, prohibit making the connection between vaccines with chronic health disorders. If an illness emerges later, of course, the doctors will say it has nothing to do with the vaccine.
3. Most vaccine safety studies do not use a true placebo.
The gold standard in medical research is the "placebo-controlled" trial. A placebo is an inactive or inert substance, such as a sugar pill or a shot of saline. In the trial, the placebo is given to one group, while the treatment group is given the experimental product. The placebo arm is used to ‘blind’ the study so the investigator doesn’t know if the subject received the Real Thing or the Inert Substance to minimize interpretation bias.
When reading a published vaccine trial, the substance used as the placebo is often not identified; it is simply called ‘placebo.’ For example, in this study for a new hepatitis B vaccine to treat chronic hepatitis B, the word ‘placebo’ is used 22 times, but we don’t know what placebo was used.
And that’s a problem. The substance used as a ‘placebo’ is often not inert; it may even may be another vaccine. For example, I remember reading a study where the meningitis C vaccine was used as a placebo because it was considered to be non-immunogenic and non-reactive. Or, in the instance of the Gardasil (HPV) vaccine, the ‘placebo’ was an injection of aluminum.
All studies for the Gardasil vaccine were said to be placebo-controlled and the total population that received a placebo included 9,701 subjects. The placebo was an aluminum adjuvant in all studies except study 018 (pre-/adolescent safety study), which used a non-aluminum-containing placebo [and we don’t know what that placebo was]
.What happened to true placebos?
The First International Ethical standards for human research evolved from the Nuremberg Tribunals in 1947 to 1949. The first principle of the Nuremberg Codes is “the voluntary consent of the human subject is absolutely essential.”
In 1964, the World Medical Association issued the Declaration of Helsinki. The document bound physicians to uphold their honor with the words, "The health of my patient will be my first consideration." The International Code of Medical Ethics declares that, “A physician shall act only in the patient's interest when providing medical care, which might have the effect of weakening the physical and mental condition of the patient.”
By 1993, the Standards had begun to change, especially when it came to vaccine clinical trials. The revised Declaration of Helsinki, Article II.3 states,
"Every patient —including those of a control group — should be assured of the best proven diagnostic and therapeutic method."
In other words, if a drug or vaccine has already been approved as ‘safe and effective,’ the use of a true placebo in the control arm of a safety trial could not readily be justified. This meant that if investigators deprived half of the trial participants of a vaccine that was approved and known to prevent illness, withholding that vaccine would be considered to be unethical.
This is also the primary justification for refusing to do a study in investigate the health of fully vaccinated vs. completely unvaccinated children. The CDC, FDA, and vaccine investigators view the unvaccinated as neglected, even abused, children that would not qualify for participation in their study because withholding vaccines is considered to be unethical.
Since its first version, the Helsinki Declaration has been amended seven times, most recently at the General Assembly in October 2013. It is now the only ‘official’ version.
This paper, published in 2014, describes in detail the ‘ethics’ of using placebos during a vaccine trial. Here are a few excerpts from the paper. I’ve modified the language a bit for clarity, as noted by the brackets:
Controversy surrounds the appropriate design of vaccine trials and, in particular, the use of unvaccinated controls (with or without a placebo) when a [similar] vaccine already exists. This paper specifies four situations in which placebo use MAY BE acceptable...
Placebo use in vaccine trials is clearly unacceptable when (1) a highly efficacious and safe [similar] vaccine exists and is currently [available] in the country in which the trial is planned and (2) the risks to participants of delaying or foregoing the available [approved/safe] vaccine cannot be [justified.]
Two years later, in 2016, new international guidelines for clinical trials involving humans were published by the Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO). This codified the rules for all future clinical trials by saying,
“As a general rule, participants in the control group of a trial must receive an established effective intervention during the trial,” meaning those in the control group **must receive** an approved vaccine so the control group can receive a benefit for participating in the trial!
Essentially this means vaccine safety trials will no longer compare a new, experimental shot to placebo for side effects, or, at the very least, all persons in the control group will get an approved vaccine when the study is closed. In essence, this eliminates the control group for long-term health comparisons.
That should blow your mind.
For those interested in digging more deeply into these rules, Guideline 5 in this document spells their issues out in detail.
Placebo is given another name
Another significant change confirmed in the 2016 Standards was that the word ‘placebo’ could be replaced by the word COMPARATOR in clinical trials or on package inserts announcing ‘We are comparing the efficacy of the new vaccine against an existing vaccine that has been approved and is therefore, safe.’
The Gold Standard of research has been compromised, twisted, even polluted. The ‘placebo’ used in vaccine research no longer needs to be an inert substance such as sterile water; in fact, it MUST include another vaccine. Inert, sterile water doesn't cause a reaction; as a substitute vaccine can. If both groups of babies in a trial have the same number of reactions, the study reports that the new vaccine “is as safe as a placebo.”
4. Vaccine-induced antibodies do not correlate with protection.
The entire vaccine industry stands on the concept of ‘safe and effective’ and the presence of vaccine-induced antibodies. However, there are many reports in highly vaccinated populations against mumps, pertussis, measles, chickenpox and now, COVID shots, that contracted the illnesses anyway. There are more, but this is a representative sample.
In 2012, an interesting article was published in the journal, VACCINE, regarding measles vaccine failure, that actually admitted the following:
“…Receiving less attention, however, is the issue of vaccine failure. While the current vaccine is acknowledged as a good vaccine, we and others have demonstrated that the immune response to the measles vaccine varies substantially in actual field use…Thus, measles outbreaks also occur even among highly vaccinated populations….This leads to a paradoxical situation whereby measles in highly immunized societies occurs primarily among those previously vaccinated.”
What Does Effective Really Mean?
The general public assumes ‘effective’ means the shot keeps you from getting sick by way of generating an antibody. But Webster's dictionary defines effective as:
Adequate to accomplish a purpose
Producing the intended or expected result
Fulfilling a specified function
By that definition, vaccines really are effective: The injection of foreign matter accomplishes the specific function and purpose it was intended to do – generate an antibody against the antigens that were injected.
Both the general public and the medical community correlate the presence of synthetically created antibodies with protection from getting sick. Experts have long taught that the body mounts an attack against pathogens through antibodies circulating in the bloodstream, known as humoral immunity. Hence, generating an antibody became the goal of vaccination and was even given the name ‘protective antibody’ to further the ruse.
But when Dr. Merrill Chase discovered the second arm of the immune system, the cell-mediated or innate immune system, in 1940, this original paradigm should have shifted. His findings later confirmed that B cells, T cells, dendritic cells, and other types of white blood cells were the body's central safeguards against infection. Before passing away in 2004, at 98 years of age, he had published at least 150 scientific papers. Two of his colleagues said about him:
''This was a major discovery because everyone now thinks of the immune response in two parts, and in many instances, it's the cellular components that are more important. Before Chase, there was only humoral immunity. After him, there was humoral and cellular immunity,” said Dr. Michel Nussenzweig, a professor of immunology at Rockefeller. ''So many areas of medicine rely on reactions that he clearly distinguished as not being antibody-mediated. People never anticipated that there would be something other than antibodies. It was an amazing finding,” said Dr. Ralph Steinman, a professor of cellular physiology and immunology at Rockefeller.
A 2001 publication, “What Are the Limits of Adjuvanticity?” states clearly:
It is known that, in many instances, antigen-specific antibody titers do not correlate with protection. In addition, very little is known about the parameters of cell-mediated immunity, which could be considered as surrogates of protection.
When the human toll-like receptors were discovered in 1997, the entire vaccine paradigm—injections to create antibodies—should have folded and gone away.
Antibody levels and protection are totally unconnected.
When one looks at the complexity of the immune system, it is perplexing how ANYONE can think an injection of foreign matter will do anything except create chaos and havoc within the intricacies of our exquisitely designed body with inborn systems designed to protect us naturally.
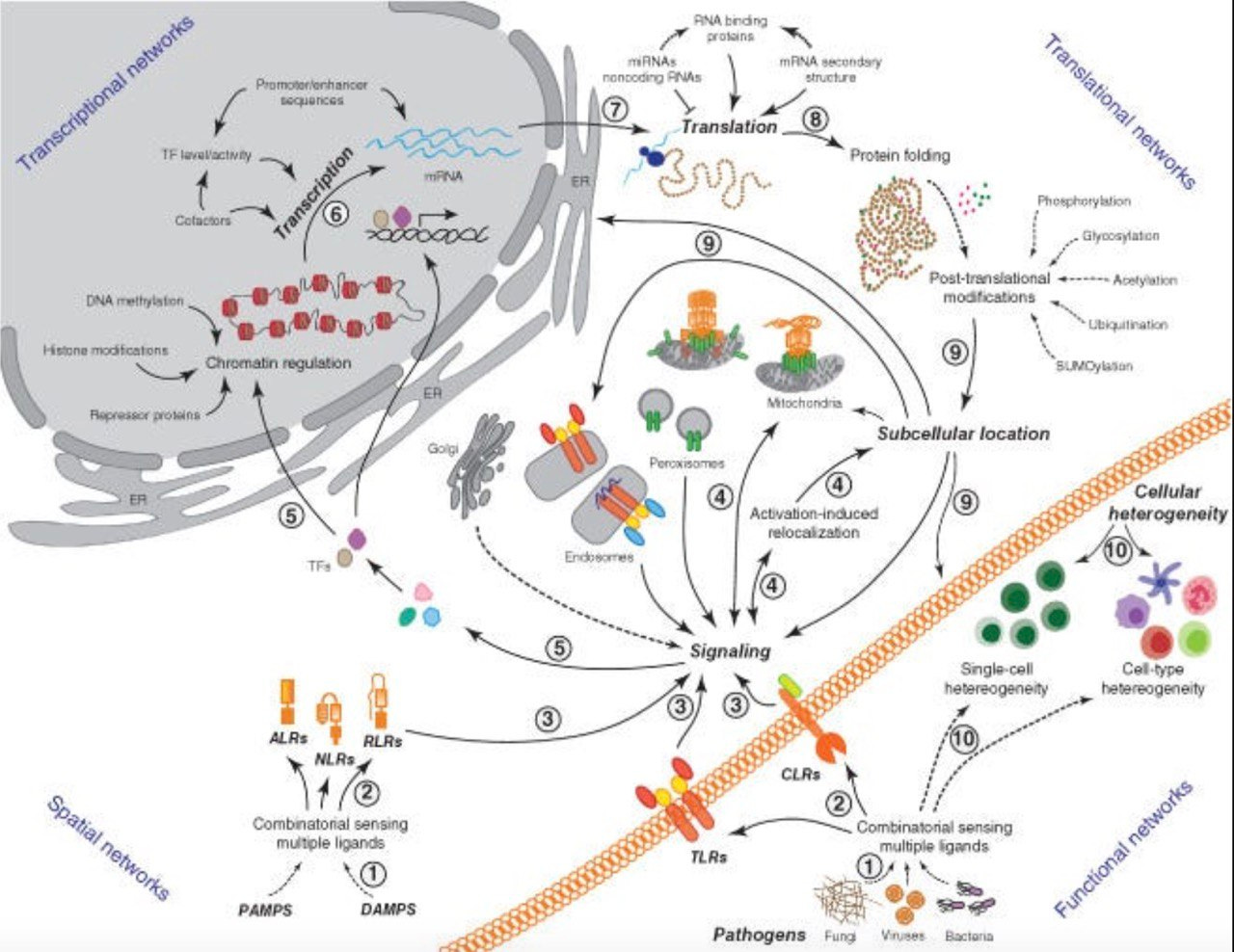
Take a look - this is an overview of what we are naturally designed to do!
The Cell-Mediated ‘innate’ Immune System
from: Network representations of immune system complexity. DOI:10.1002/wsbm.1288
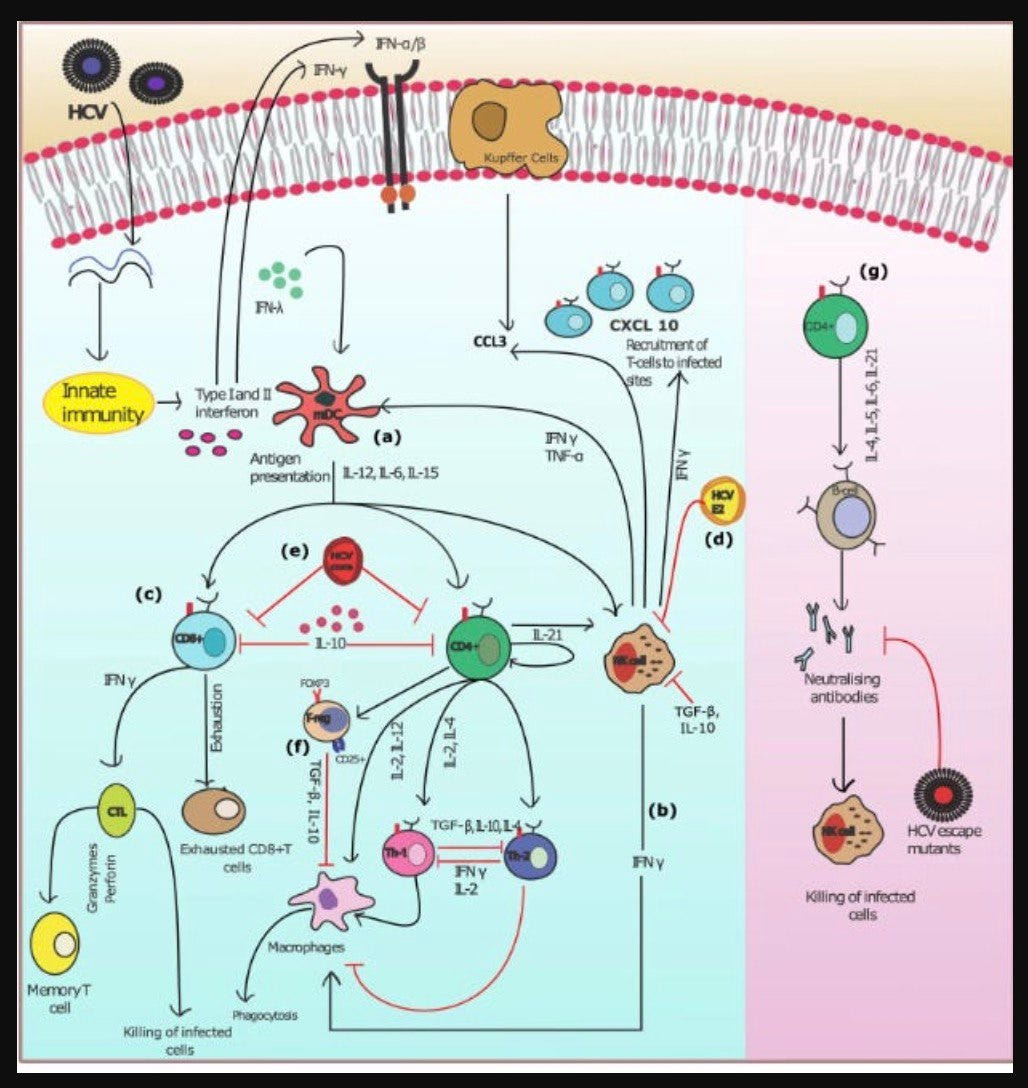
The Humoral ‘adaptive’ Immune System
From: Model of the adaptive immune response system against HCV infection reveals potential
immunomodulatory agents for combination therapy. DOI: 10.1038/s41598-018-27163-0
Conclusion
For more than 200 years, vaccination has been accepted as safe, effective, and protective. It is none of these. The shots can be described as a medical sacred cow, which is defined as, "a medical procedure unreasonably immune to criticism."
The strong responses generated when it is determined these “old cows should be sacrificed” are called heresy and disinformation. We dare not call out, or even suggest, that the status quo is wrong.
When Copernicus insisted that the sun, not the earth, was the center of the solar system, his theory went against the philosophical and religious beliefs held during those times. When two other Italian scientists, Galileo and Bruno, embraced Copernican theory after his death, their comments were also considered blasphemous.
Bruno was tried before the Inquisition, condemned, and burned at the stake in 1600. Galileo was later brought forward and, in front of his "Betters," was forced to renounce his beliefs under the threat of torture and death. Even after his confession, he was sentenced to imprisonment for the remainder of his life.
The more one investigates all of the vaccinations - including the present mRNA covid-19 injections - the more one discovers the scientific distortions and frank lies. But speaking out against the status quo these days has become dangerous, akin to being a Copernican heretic.
If I am called a heretic for exposing the truth about this multi-generational deception, I am glad to be in good company.










Dr. Tenpenny, you are a refreshing breath of air. Thank you for being a rare source of truth in a world so full of lies.
"It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of The New England Journal of Medicine." Dr. Marcia Angell